Edition 01 | January 2025
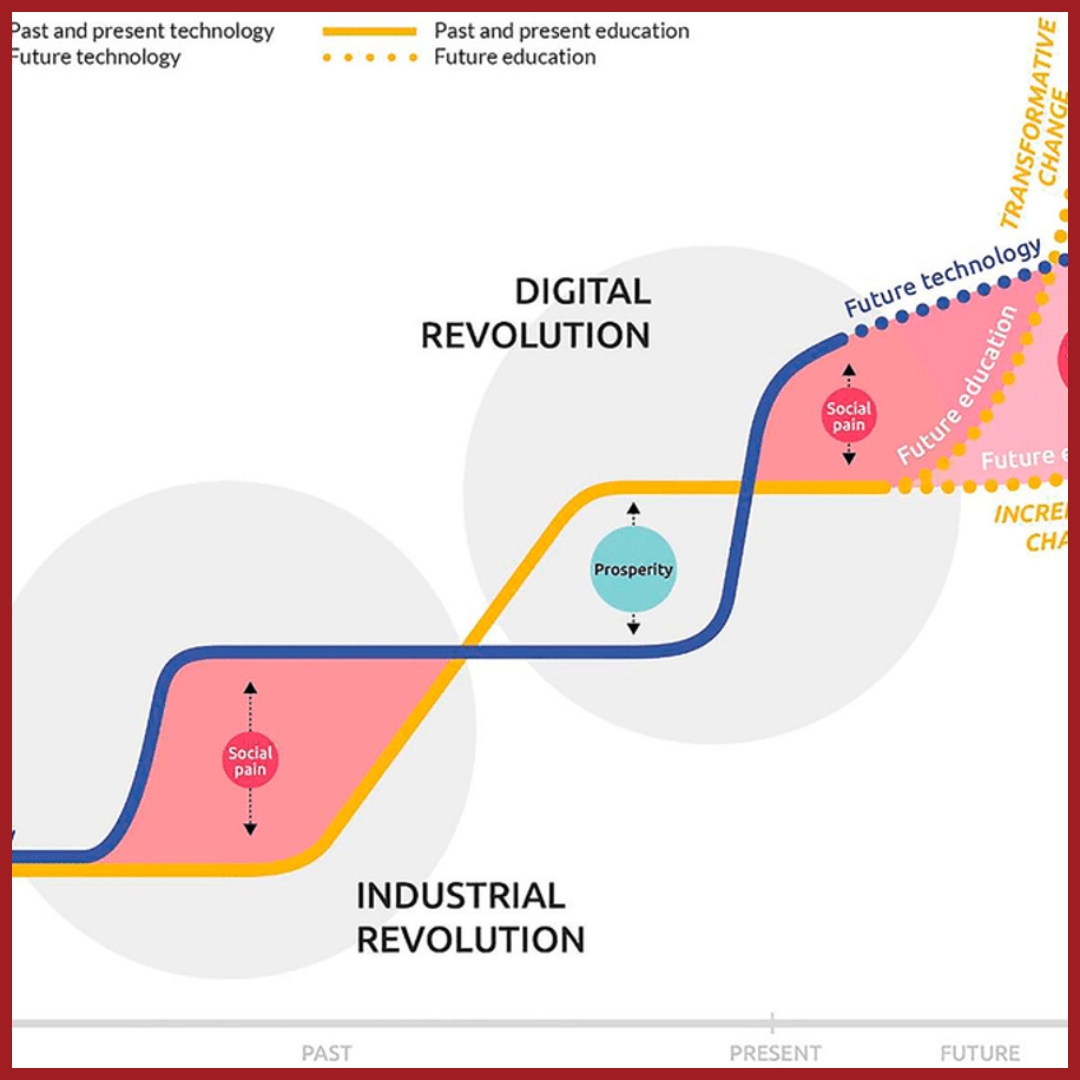
From Learning Data to Classroom Instruction
Explore how data-driven insights can transform teaching by addressing misconceptions and fostering deeper understanding.
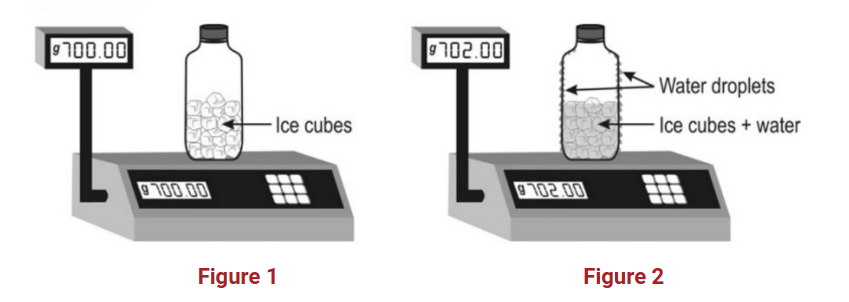
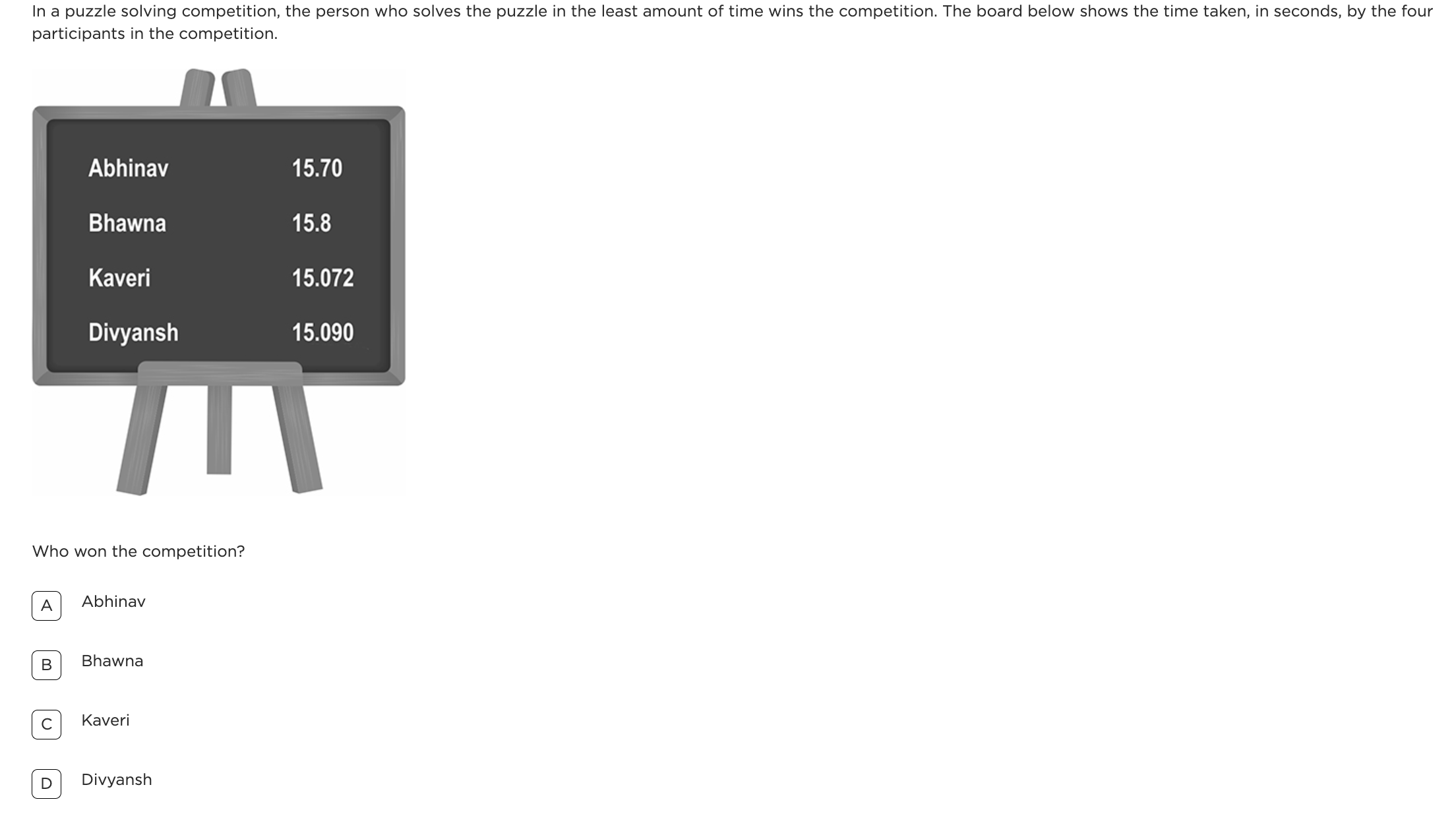
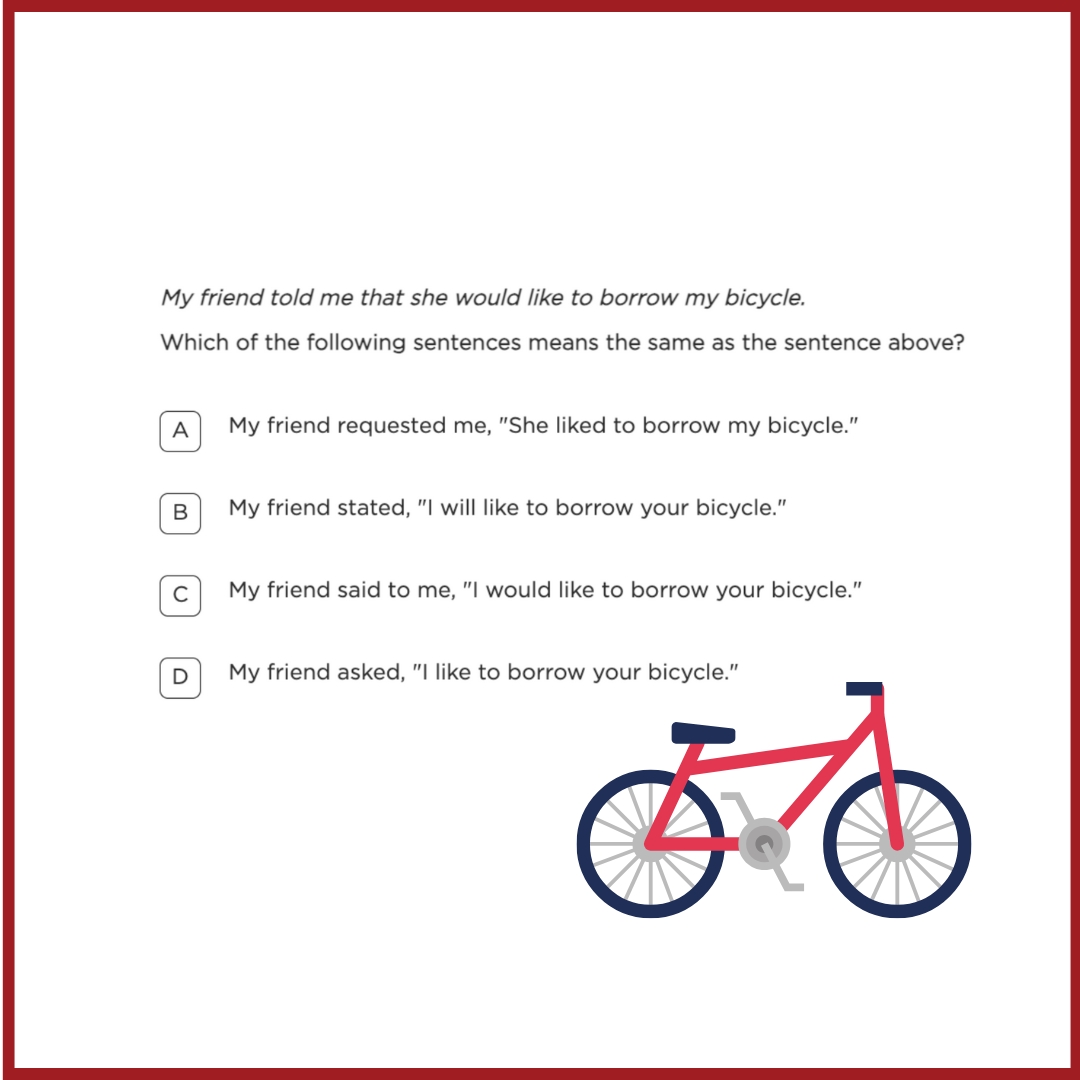
Question: Kevin placed some ice cubes inside a glass bottle and then closed the bottle tightly. He placed the bottle on a weighing balance, as shown in the figure 1. He saw water droplets forming on the inside and on the outside of the bottle and that the weight of the bottle kept increasing as shown in the figure 2.
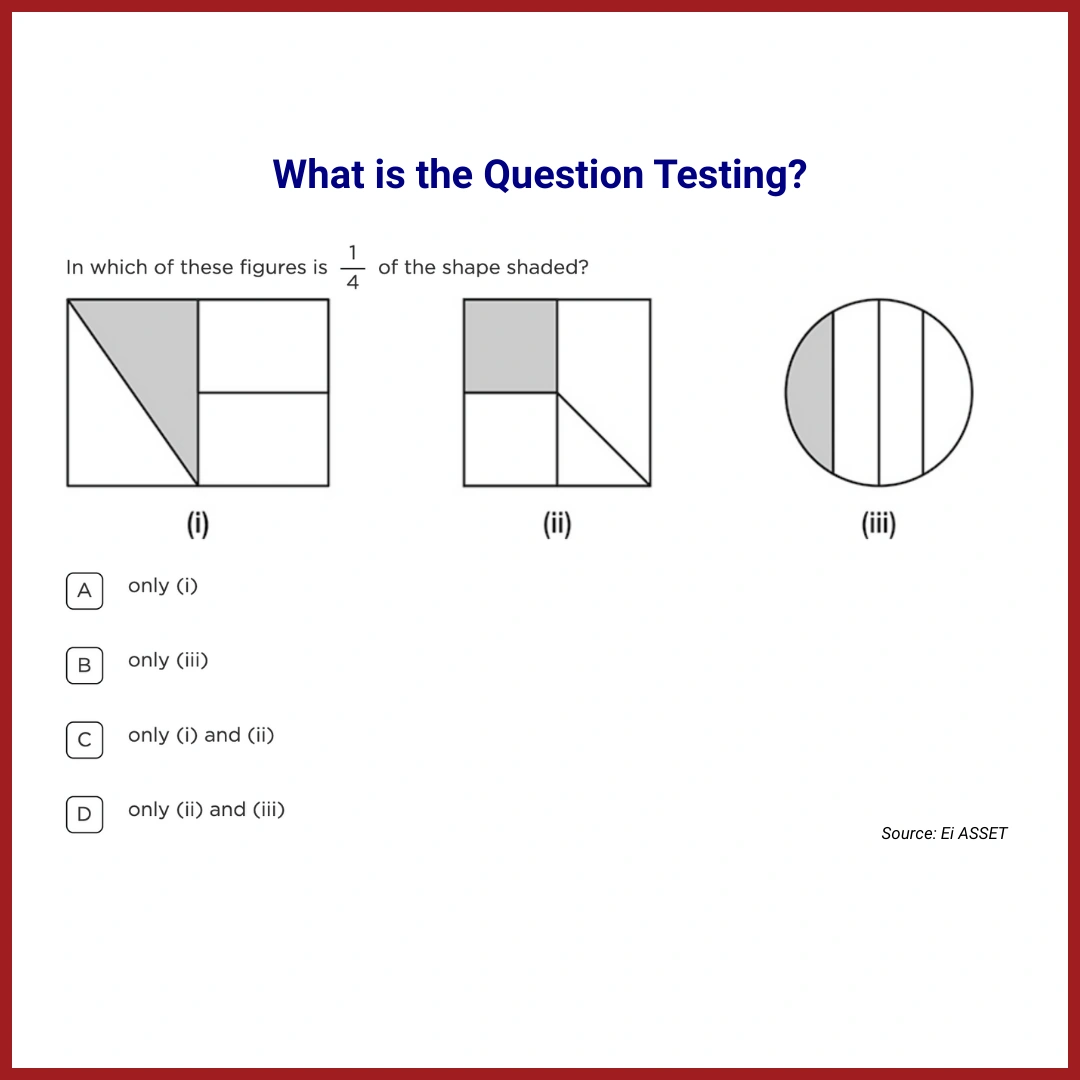
What is the Question Testing?
This question is testing students’ understanding of condensation and mass conservation. Specifically, it requires students to realise that when water droplets form on the outside of a cold bottle, they originate from water vapour in the surrounding air. This leads to an increase in the weight of the bottle because the condensed water is now part of the bottle’s weight.
What is the Most Common Wrong Answer and Possible Misconception?
What Will Happen if Children Do Not Show Development of This Concept?
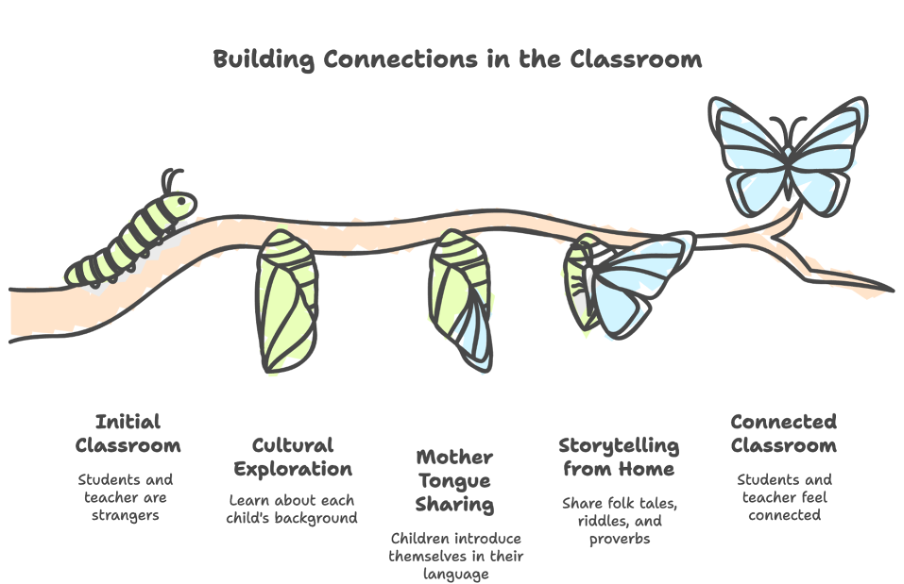
How Should I Remediate This in My Class?
Enjoyed the read? Spread the word
Interested in being featured in our newsletter?
Check out the latest edition here.
Feature Articles
Join Our Newsletter
Your monthly dose of education insights and innovations delivered to your inbox!
powered by Advanced iFrame